Material improvement is an essential prerequisite for the use of renewable energy in large scale.
Production of hydrogen by electrolysis of water using renewable energy is expected to become increasingly important in the closest future. Currently, it is normally more expensive to produce hydrogen by water electrolysis than by gas reforming. Today it is only produced around 5% of world hydrogen by electrolysis. A significant reduction in the price of hydrogen produced by electrolysis coupled with rising prices for oil / gas could change this. An essential element here is that the efficiency of the electrolysis process still has a great potential for improvement.
Research activity within hydrogen production by electrolysis of water has until now been remarkably small, compared with research activity in fuel cells. This despite the fact that hydrogen produced by renewable energy is a prerequisite for the use of fuel cells could possibly lead to a reduction in the use of fossil fuels. Likewise, water electrolysis is crucial for wind, wave, hydro and solar energy, which can be used to produce fuels for transport as well. There is thus an area which is relatively open, both in research and for commercialization.
Technology developed for fuel cell research can also be used for water electrolysis.
An alternative to the conventional electrolysis technology is the use of the technique known from Proton Exchange Membrane (PEM) fuel cells, which uses proton conductive polymer membranes as electrolytes. It simply saying is about reversing a fuel cell, i.e. impose a voltage so that it "runs backwards" and then develops H2 and O2 from water, instead of burning H2. The principles of PEM fuel cells are described under research project "Fuel Cells". Because of the anodic oxygen evolution reaction (OER) in cells by electrolysis, the electrode, however, has to be more resistant than required by fuel cells and carbon / graphite cannot be used anymore for this purpose. There is thus a number of material problems (high cost) associated with PEM electrolysis technology in its current form, and this has limited its use compared to conventional alkaline electrolysis. On the other hand, such systems can operate at much higher temperatures, up to 150 °C or even 200 °C. Not least therefore they are expected to achieve much higher efficiency since a higher operating temperature means that the required energy (ΔG) for operating water decomposition process decreases. Another approach includes developing of anion conduction membranes, which can operate in Alkaline Water Electrolysis (AEC) cells, where conventional Nickel and Nickel oxide electrodes can be utilized instead. Then, the advanced coatings of such electrodes become an attractive field for further development of the efficiency enhancement.
A scheme of a water electrolyzer operating at elevated temperature.
New materials technology must be developed: Advanced surface coatings.
As shown above there is a need for new electrode materials, especially at the anode side, which both meet the technical requirements, and simultaneously is are efficiently cheap. There are, of course, in addition, a number of other issues also may be subject to further research and development, not least new cheaper catalysts are not based on precious metals, and new polymer membranes with higher conductivity etc, just as in fuel cells.
Anodes (providing OER) need developing of new materials and advanced surface coatings that are sufficiently chemically resistant to water vapor and oxygen gas mixture, combined with the high anodic potential at the electrode during water electrolysis. These surfaces must also possess sufficient electrical conductivity and electrochemical activity while keeping overpotential at low lever combined with high values of current densities.
In our research on electrode materials for water electrolysis at DTU Energy Conversion, we plan to test various refractory metals (Ta, Nb, Ti, Zr or Mo) in solid form or as their coatings, as well as their carbides and nitrides.
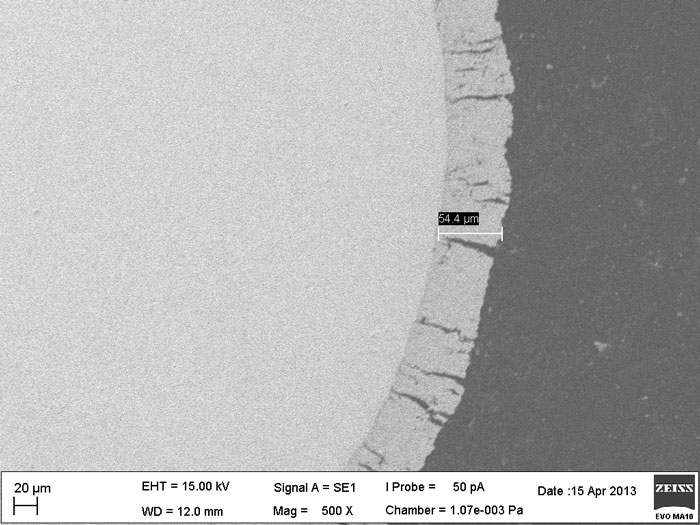
Scanning electron microscope image of dense tungsten carbide coating made on tungsten wire – a new generation electrode for water electrolysis
The assignments are offered in the manufacture of coatings, electrochemical and physical characterization and their test in electrolytic cells (New electrode materials for hydrogen production by water electrolysis). The work is closely linked to current research in fuel cells.
External partners
The section has had a longstanding collaboration with Danfoss A/S regarding coatings with refractory metals, especially tantalum, which has resulted in the creation of a new company, Tantaline, which continues to collaborate with our group. Among other partners are: Siemens A/S (alkaline anodes), GreenHydrogen.dk (alkaline electrolysis cells), Technical University of Munich (advanced coatings preparation).
For further information contact: Niels J. Bjerrum, Erik Christensen, Jens Oluf Jensen, Jens von Barner, Irina Petrushina or Aleksey Nikiforov