Many materials, such as the battery alloy, LaNi5, are able to absorb and contain hydrogen. When heated, the hydrogen is desorbed and can be used in fuel cells or directly in internal combustion engines. Many hydrogen storage materials are already in use today, however they do not satisfy the need for the future in terms of energy demand. There is a great need to develop and discover new and better materials for storing hydrogen.
Many factors join together to define a hydrogen storage material that is practical.
It should:
- Absorb hydrogen quickly
- Absorb hydrogen at pressures between 1 and 10 atm
- Be able to contain a high amount of hydrogen, at least 6 wt.%
- Desorb hydrogen at temperatures below 200°C
Furthermore, it must be resistant to degradation from cycling and reaction with the atmosphere.
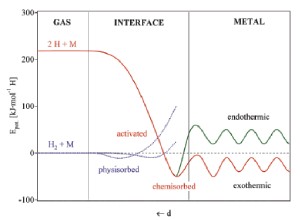
Traditional metal hydrides
Up until the end of the millennium, the focus of hydrogen storage material research has been mostly on alloys, such as LaNi5, Mg2Ni, and TiFe. In these materials, gaseous hydrogen interacts physically with the surface and enters into the metal crystal lattice, effectively creating a hydride phase.
Complex hydrides
In 1996, it was discovered that hydrogen can be released reasonably fast at temperatures below 200°C from titanium doped sodium alanate, NaAlH4.
This led to a shift in focus of hydrogen storage material research to the so-called complex hydrides. A few years later, in 2002, it was published that a system consisting of LiNH2 and LiH could reversibly contain above 6 wt.% hydrogen at temperatures close to 200°C. New systems, substituting the metal cations and doping the materials have since been developed.
In our group, we have ongoing research to develop the complex metal hydride systems. This includes exploring ways to destabilize systems to operate at lower temperatures and make them more stable.
On-site Apparatus
Synthesis of new materials:
- Ball-milling for mechanical alloying
- Gloveboxes with inert atmosphere
- Dry-air glovebox for purification with organic solvents
- High temperature ovens
- Vacuum ovens
Characterization of materials:
- Thermal gravimetric analysis
- Differential scanning calometry
- Differential thermal analysis
- Online mass spectrometer
- High pressure microbalance in inert glovebox
- Scanning Electron Microscopy
In collaboration with other laboratories:
- Arc melting
- Melt spinning
- X-ray diffraction (ex situ and in situ)
- Small Angle X-ray Scattering (ex situ and in situ)
- Transmission Electron Microscopy
- Sievert apparatus for hydrogen absorption/desorption
- Small Angle Neutron Scattering
- RITA-II triple axis neutron spectrometer
|


|
Collaboration
Our group collaborates with a large network of research groups within the field of hydrogen storage reasearch including:
- Risø, Denmark
- University of Iceland
- Stockholm University, Sweden
- Uppsala University, Sweden
- University of Oslo, Norway
- Sandia National Laboratories, USA
Participation
We welcome students of all levels to do special courses, bachelor, mid-term, and exam projects. Examples of subjects include:
- Theoretical studies including making reviews of current literature
- On the role of titanium in sodium alanate for hydrogen storage
- Synthesis of transition metal alanates
- Metal amide hydrogen storage systems
- Experimental studies on conventional and complex hydrides
- Substitution of Ca with Mg in Ca-Ni systems
- Destabilization of the LiH-LiNH2 system
- Synthesis and characterization of transition metal borohydrides
For more info, please contact: Jens Oluf Jensen